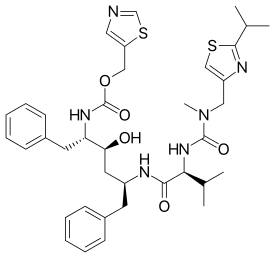
Lopinavir/Ritonavir as Single-Drug Therapy for Maintenance of HIV-1 Viral Suppression1. Hypotheses, predictions, and scientific theories
2. Sample size and data in error analysis 3. Scientific methodology, including discovery science, model building and testing, hypothesis testing, and engineering |
Clinical Trial
|
| ||||||